Thesis and Research Summary
Dr. Kevin S. Kolack
Professor K completed his Ph.D., "From Biochemistry to Molecular Materials: A New Perspective on Transition Metal Carboxylates," in inorganic chemistry in Professor George Christou's research group at
Indiana University in May 1997.
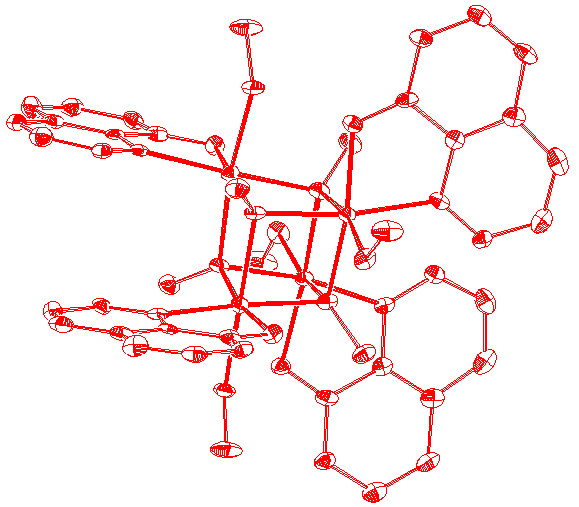
Diagrams of two of
the newer molecules presented in the thesis are given below, along with the table of contents and acknowledgments.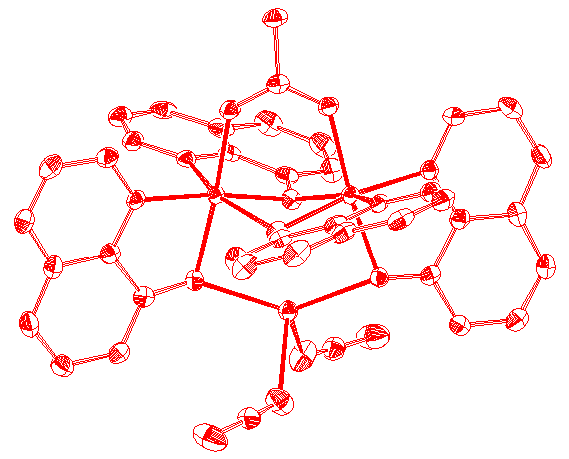
The first man of science was he who looked into a thing, not to learn whether it furnished him with food, or shelter, or weapons, or tools, or armaments, or playwiths but who sought to know it for the gratification of knowing.
-- Samuel Taylor Coleridge
Try to learn something about everything and everything about something.
-- Thomas Henry Huxley
Since my full-circle return to Bloomington in 1991 (I was conceived here in 1969!), much has changed and much has remained the same. There are a few people I must thank for this. My mother and my brother have been an unwavering source of support and love that I could not have done without. Three men have served as older and more mature (?!) role models through their incredible devotion to both family and work that I have tried to emulate in all of my activities: Dr. David H. Kolack, my father (whom I miss very much), the best optometrist and father the world has ever known; Mr. Dexter Mills, my high school principal; and Mr. Faron Livingston, Chief of the Bloomington Township Department of Fire and Emergency Services.
Good fortune has allowed me to live a life where I could interact and learn from a number of groups of people. The volunteers and employees (especially Mr. Marc Presti and Mr. Russell Edwards) at the fire department have been a second family to me, and it is largely their involvement in my life that has led me to call Bloomington my home. Speaking of second families, special thanks to lifelong friends Mr. Jimmy Easterly and Mr. Mike Kendrick and their families for always being there. All the participants and staff (especially Ms. Maggie Mays, Professor Boris Leskin, and Mr. Robin Selfridge) of the Beginningsä workshop deserve special thanks for awakening in me more feelings than I can put into words. I can never thank Mr. Peter Sklar enough for founding this program and choosing me to be a part of it. My friends and colleagues in the Department of Chemistry at Indiana University have been wonderful, especially Dr. Chuck Crawford, Dr. Vince Grillo, Dr. Scott Haubrich and Dr. Mike Wemple, with special recognition going to Dr. Milissa Bolcar and Mr. Guillem Aromí for proofreading this text.
Life is a series of lessons, and although some people now call me "professor" and though it is a bit unconventional, I would like to acknowledge the efforts of a few more members of the public school system in Georgia who have provided me with some vivid memories of "formal" schooling: my first grade teacher Mrs. Green at East Side Elementary; my fourth grade teacher Mrs. Donnelson; my eighth grade English teacher, Mrs. Duncan, who taught me how to write (diagramming sentences is important even for chemists!), and whose efforts were supported later in the ninth grade by Mrs. Brown and in tenth and twelfth by Mrs. Lee, despite their southern accents. This list would not be complete without mention of two more people critical to my upbringing: my senior year band instructor, Mr. John Abert, who reminded us that excellence and humor are not mutually exclusive; and my AP History teacher, Mrs. Linda Morrison, perhaps the best teacher I have ever known– from her, I have taken much of what I now give my own students, and for this I shall always be grateful. I would also like to thank the outstanding chemistry faculty at The University of Virginia for their excellent instruction, most importantly my advisor, Professor Frank Carey, and my research director, Professor Ian Harrison. Each of my Buddhism professors at UVA also deserves special thanks. Finally, no list of teachers would be complete without recognizing the support of my research advisor at Indiana University, Professor George Christou. With his approval, I was able to pursue research new to the group, both in and outside the lab. I am indebted.
(final paragraph omitted)
Kevin S. Kolack
From Biochemistry to Molecular Materials: A New Perspective on Transition Metal Carboxylates
Abstract
An improved synthetic methodology for production of the trinuclear manganese complexes [Mn3O(O2CCH3)6(pyr)3]z+ (z = 0, 1) is reported. Several long chain fatty acid derivatives of the basic metal carboxylate structure are synthesized and structurally characterized. Addition of nBu4NMnO4 to a mixture of Mn(O2CCH2CH3)2 and NaClO4 in ethanol, acetic acid and pyridine produces [Mn3O(O2CCH2CH3)6(pyr)3](ClO4) (1). Removal of acetic acid as a toluene azeotrope from [Mn3O(O2CCH3)6(pyr)3](ClO4) in the presence of a stoichiometric amount of valeric acid produces [Mn3O(O2C(CH2)3CH3)6(pyr)3](ClO4) (2). When 4-octyloxybenzoic acid and Cr(ClO4)3 are heated in pyridine, single crystals of [Cr3O(O2CC6H4-p-O(CH2)7CH3)6(pyr)3](ClO4) (3) are obtained. The complex [Fe3O(O2CR)6L3] (R = (CH2)6CH3; L = H2O, HO2CR) (4) results from combination of aqueous sodium octanoate and an acetone solution of FeCl3· 6H2O.
A new decanuclear manganese aggregate is synthesized and structurally characterized. Mn10O2(O2C(CH2)3CH3)18(H2O)4 (6) results from the addition of peracetic acid to an ethanol mixture of Mn(O2C(CH2)3CH3)2.
Several 8-hydroxyquinoline complexes of nickel are also reported in which the ligand adopts rather rare binding modes. Reaction of Ni(O2CCH3)2· 4H2O with 8-hydroxyquinoline and NaOH in MeOH affords a green precipitate of Ni4(OMe)4(hqn)4(MeOH)4 (8). The structure is of the distorted cubane motif common to transition metal alkoxide chemistry and previously reported by this group for a dibenzoylmethane analog. The solid-state variable-temperature effective magnetic moment of 8 is in agreement with the D2d symmetry of the [Ni4(OMe)4]4+ core, modeled by a two-J equation giving J1 = -10.5 cm-1, J2 = 15.0 cm-1, g = 2.04 and q = 2.11 K. For the analogous reaction in acetonitrile, Ni2(hqn)4(Na)(MeCN)2 (9) is obtained, and an identical ethanol adduct (10) is produced when the reaction is performed in ethanol. The temperature dependent magnetic data of 9 can be fit to a one-J model, giving J = -0.13 cm-1 and g = 2.04. The unusual cis conformation of Ni(hqn)2(pyr)2 results from the reaction in water and recrystallization of the product from pyridine. When nickel perchlorate is used as the metal source and the reaction is performed in ethanol, the distorted cubane [Ni4(OH)(hqn)3(hqnH)3(EtOH)3][ClO4] (12) results. The cubane core of 12 is of C3v symmetry, and the magnetic data is fit to a fundamentally different two-J model, giving J1 = 0.93 cm-1, J2 = 3.82 cm-1, g = 2.298 and q = -2.12 K. The magnetic data of 8, 9 and 12 follows Hatfield-Hodgson type magnetostructural correlations between J and Ni-O-Ni angle for nickel dimer and cubane complexes.
Table of Contents
| Acceptance | ii |
| Dedication | iii |
| Acknowledgments | iv |
| Abstract | vi |
| Table of Contents | viii |
| Abbreviations Used | x |
| List of Structures | xi |
| List of Tables | xii |
| List of Figures | xiv |
| Introduction | |
| I.1 Magnetism | 5 |
| I.2 Biochemical Relevance | 7 |
| I.3 Research Goals | 10 |
| I.4 References | |
| Chapter One | 13 |
| 1.1 Introduction | 14 |
| 15 | |
| 16 | |
| 1.2 Results and Discussion | 19 |
| 19 | |
| 21 | |
| 21 | |
| 32 | |
| 40 | |
| 52 | |
| 62 | |
| 62 | |
| 67 | |
| 71 | |
| 72 | |
| 73 | |
| 73 | |
| 74 | |
| 1.3 Conclusions | 75 |
| 1.4 Experimental | 76 |
| 1.5 References | 81 |
| Chapter Two | 87 |
| 2.1 Introduction | 88 |
| 2.2 Results and Discussion | 89 |
| 89 | |
| 91 | |
| 91 | |
| 96 | |
| 106 | |
| 2.3 Conclusions | 108 |
| 2.4 Experimental | 108 |
| 2.5 References | 112 |
| Chapter Three | 115 |
| 3.1 Introduction | 116 |
| 3.2 Results and Discussion | 119 |
| 119 | |
| 120 | |
| 120 | |
| 125 | |
| 130 | |
| 135 | |
| 140 | |
| 146 | |
| 164 | |
| 164 | |
| 3.3 Conclusions | 165 |
| 3.4 Experimental | 165 |
| 3.5 References | 170 |
| Curriculum Vita | |